Abstract
Introduction: The survival of double refractory multiple myeloma (MM) patients was poor in the pre- monoclonal antibody era, with a median overall survival (OS) of 9 months only.
Daratumumab used as a single agent has shown significant efficacy resulting in an overall response rate (ORR) of 30%, and OS of 20 months in patients, failing immunomodulatory drugs (IMIDs) and proteosome inhibitors (PIs). Daratumumab combined with pomalidomide and dexamethasone has yielded ORR of 60% and median OS of 17.5 months in such patients. Daratumumab has been recently introduced to the early relapsed MM setting, providing significant improvement in progression-free survival when administered in combination with IMIDs or PIs.
The current retrospective study has evaluated the characteristics and outcome of MM patients who had progressed while being receiving daratumumab, aiming to define prognostic factors and optimal therapeutic approaches for this patient population.
Methods: MM patients treated with daratumumab alone or in combinations in 11 Israeli centers between 01.2014 and 07.2018, all experiencing disease relapse/progression were included. Data including demographics, disease-related parameters at diagnosis [MM type, extramedullary disease (EMD), ISS (International Scoring System), LDH level high-risk cytogenetics], prior treatment regimens, response duration to the last pre-daratumumab therapy, treatment and outcomes post-daratumumab failure were analyzed.
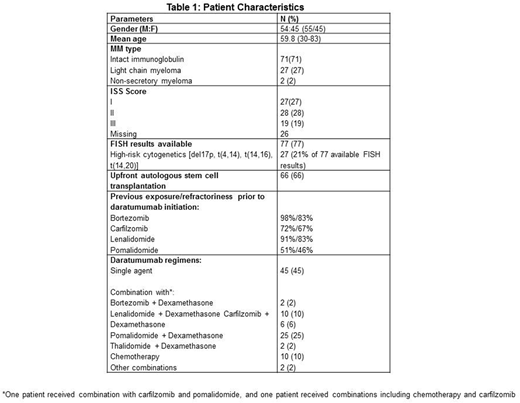
Results: One hundred consecutive patients progressing on daratumumab were included in the study. Patient characteristics are presented in table 1. Daratumumab was used in 2nd-3rd line therapy in 17 patients (17%), 4th in 22 patients (22%) and 5th-9th line therapy in 61 patients (61%).
Sixty four patients (64%) were refractory to at least 3 novel agents before starting a daratumumab combination; 36 (36%) of them were quadrate refractory to bortezomib, carfilzomib, lenalidomide and pomalidomide. Forty five patients (45%) received daratumumab as a single agent and 55 (55%) as a combination therapy (table 1).
Median duration of response to the last pre-daratumumab therapy was 5 months, with duration of ³6 months predicting response to daratumumab (P=0.019). Fifty seven percent of patients achieved stable disease or better with daratumumab combinations, with an ORR of 38% [partial response (PR) or better] and 15% achieved very good PR (VGPR) or complete response. Median time to progression on daratumumab was 3.1 months. It was shorter in patients treated with daratumumab as a single agent than in those receiving a combination therapy (2.5 vs 4.7 months, p=0.012). Progression (n=13) or de novo (n=19) EMD was recorded in 32 patients (32%).
At time of relapse/progression, daratumumab was stopped in 45 patients (45%), and continued in combination with other agents in 33 patients (33%). Data regarding actions taken post-daratumumab failure were unavailable for 22 individuals. In 58 patients, for whom data regarding response to a post-daratumumab regimen were available, the ORR was 34%.
Median follow-up after daratumumab failure was 8 months (0 -33.5 months), with a median OS of 5.3 months; 25% of the patients survived <2.2 months and another 25% - >14.1 months. Notably, daratumumab given in combination with chemotherapy was associated with a worse prognosis (HR=2.7, P=0.007). No OS difference was found between those who stopped daratumumab at time of failure and those who continued it. Age, gender, high-risk cytogenetics and lines of previous therapies did not affect OS in this patient group.
Longer duration of response to both pre-daratumumab and daratumumab therapy was found to be associated with a prolonged OS after daratumumab failure (HR=0.929, P=0.006 and HR=0.872, P=0.024, respectively).
Conclusions: The prognosis of double refractory MM patients, failing daratumumab therapy, is poor, with a median OS of 5.3 months. Post-failure continuation of daratumumab in a different anti-myeloma combination has not improved OS. Durable responses to both pre-daratumumab and daratumumab therapy are both associated with a superior OS in patients progressing on daratumumab, most probably reflecting a more favorable disease biology. Given that most patients in this study have been heavily pretreated, further evaluation of treatment strategies in patients who fail daratumumab combinations at earlier disease stages is warranted.
Cohen:Janssen: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Takeda: Honoraria, Research Funding. Tadmor:ABBVIE: Consultancy; JNJ: Consultancy; NOVARTIS: Consultancy; PFIEZER: Consultancy; ROCHE: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal